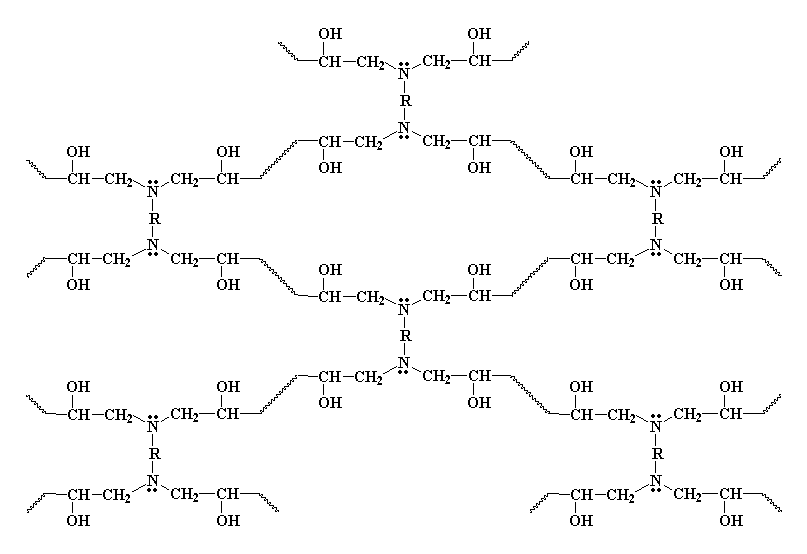Composition and Structure: The Molecular Backbone
The formation of the cross-linked and cured structure of epoxy resin begins with the mixing of two main components: the epoxy and the curing agent. Epoxides are organic compounds containing epoxy groups, which are highly reactive due to their cyclic oxygen ring. When mixed with the curing agent, typically an amine or acid, a chemical reaction known as addition polymerization occurs. During this process, the epoxy groups open and bond with the groups from the curing agent, forming a three-dimensional cross-linked network.
Why Does Molecular Structure Matter?
The molecular structure of epoxy resin provides it with a dense and tightly packed network. This network acts as a formidable barrier against the intrusion of chemical agents. Imagine a fortress wall built with bricks tightly interlocked; similarly, the tightly bound epoxy molecules repel the penetration of chemical substances, preserving the integrity of the material.
The factors that make cross-linked epoxy resin highly chemically resistant
The effect of the dense and resilient polymeric network acting as a physical barrier
In the image above, we can observe the cross-linked structure of epoxy paint from a flat or two-dimensional perspective. In reality, the matrix is three-dimensional. As we can see, it is a crystalline and dense structure, formed by pores (the gaps between chains) that, with good chemical synthesis, can become very small. The smaller these pores are, the more impermeable the matrix will be to the entry of external gases and liquids that could chemically attack the structure. This is known as the physical barrier effect. Additionally, besides allowing less particle inflow, the pore channels often lead nowhere, trapping substances in the porous labyrinth of the physical barrier. Although there are not many chemically vulnerable sites in the network (as all are formed by strong covalent bonds), extremely harsh conditions with very aggressive chemicals, high temperature, and high pressure could eventually degrade the paint to some extent. However, thanks to the existence of this physical barrier effect, the chances of this happening are significantly reduced.
Formed by strong covalent and sigma bonds
The epoxy polymer network, unlike other thermoplastic paints, is structurally composed of the strongest and most resilient chemical bonds, which are covalent bonds. These bonds are approximately 4 times stronger than hydrogen bonds and 20 times stronger than Van der Waals bonds. Additionally, all bonds in this network are single and sigma bonds. Covalent bonds can be sigma or pi bonds; pi bonds are weaker than sigma bonds and are found in double and triple bonds. However, this network consists only of single and sigma bonds, which makes it highly resistant and chemically inert.
Electronic resonance of the benzene groups that provide chemical stability to the matrix
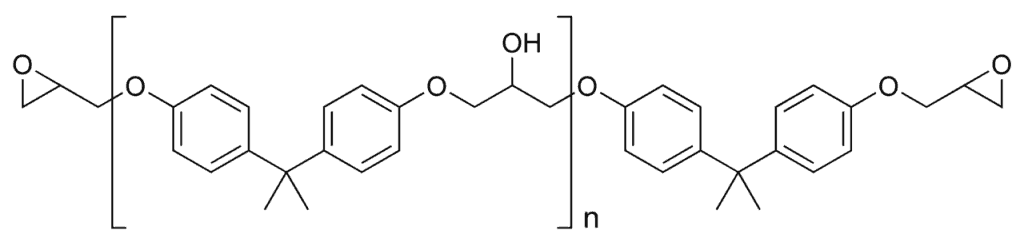
The epoxy network has the particularity of containing benzene rings within its chains, which are the hexagons shown in the image below. The image displays a single epoxy polymer chain. Each of these chains contains 2 benzene rings. Therefore, when we add up all the chains that can be present in a volume of paint, we find a huge number of benzene rings. These rings have the particular characteristic of exhibiting chemical resonance, a property that provides great stability to this specific chemical group. Resonance allows the pi electrons (double bonds) of benzene to delocalize over the entire benzene ring, enhancing the stability of the group. This prevents the existence of fixed partial charges at any site that could be vulnerable to attack. Whenever electrons arrive at a part of the benzene ring and potentially turn it into a nucleophilic and vulnerable area, these electrons automatically redistribute and leave that part as stable as it was before